Carnot Cycle Which of These Following Statements Is True
B It is a cycle in which the net heat is the same as net work. A Carnot heat engine is a theoretical engine that operates on the reversible Carnot cycle.

Chapter 5 The Second Law Of Thermodynamics Updated 7 5 2014
C the cycle binds carbon atoms from carbon dioxide into organic compound.

. An actual engine is to be designed having same efficiency as the Carnot cycle. Density p versus temperature T graph of a thermodynamic cycle of an idea gas is shown. A Carnot Cycle consists of reversible processes only The Carnot Cycle is not a realistic model for real vapor power cycles The Carnot Cycle cannot be approximated using actual devices.
A refrigerator is basically a heat pump used to pump the heat absorbed from the material at lower temperature inside the refrigerator into surrounding at higher temperature The coefficient of performance of a refrigerator is defined as ratio of heat removed Q 2. A Carnot cycle is a theoretical ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. In the early 1820s Sadi Carnot 17861832 a French engineer became interested in improving the efficiencies of practical heat engines.
Which of the following statements are true regarding the Carnot Cycle. All four of the processes are adiabatic. All the heat engines are based on Carnot cycle.
Check all that apply. If BC and AD are the part of rectangular hyperbola then which of the following graphs will represent the same thermodynamic cycle. The condenser over cools the liquid vapor mix.
It may be stated in the following forms. Any system working between T1 hot reservoir and T2 cold reservoir can never have more efficiency than the Carnot engine operating between the same reservoirs. The ideal Rankine cycle has lower efficiency than a Carnot cycle operating between the same two temperatures.
Possible but with lot of sophistications. It provides concept of maximising work output between the two temperature limits. D The Carnot cycle is the basis for climate change.
All of the above. A 4 isothermal processes b 4 adiabatic processes c 2 isothermal and 2 adiabatic processes d none of the mentioned Answer. We know that carnot cycle is reversible cycle and reversible cycle has maximum efficiency of all other cycles and so only statement A is incorrect Video Explanation Solve any question of Thermodynamics with-.
All of the following statements about the Carnot cycle are True EXCEPT. What is true of the Carnot Cycle. A No engine can be more efficient than a perfectly reversible engine working between the same two temperatures.
Since carnot cycle works between two constant temperature and heat transfer is taking place at constant temperature. A reversible cycle has following processes. In real life it is nearly.
B The total internal energy change around a complete Carnot cycle is negative meaning it does work or expels heat. The cycle that operates in the counterclockwise direction on a T-s diagram of a Carnot cycle is called a reversed Carnot cycle. Two of the processes are isothermal.
AIt is a cycle in which the efficiency is independent of the working substance. 1 an adiabatic expansion that gives the gas 200 times its initial volume 2 a constant-volume process 3 an isothermal compression back to the initial state of the gas. B its efficiency depends only on the absolute temperature of the hot reservoir used.
Which of the following is the primary reason the efficiency is lower. B The efficiency of all reversible engines working between the same two temperatures is the same whatever the working substances. A three-step cycle is undergone reversibly by 400 mol of an idealgas.
The pump is not reversible. The basic model for this engine was. The working fluid is air.
It is used as the alternate standard of comparison of all heat engines. Carnot cycle is a reversible cycle. A heat pump is a heat engine working in reverse direction.
True A Heat engine receives 5 MJ heat transfer at 995 K gives out 2 MJ as work and the rest of the heat to the surrondings at 25C. C It is a cycle modeled by a closed system. C The Carnot cycle can be analyzed to prove that entropy is a state function.
In 1824 his studies led him to propose a hypothetical working cycle with the highest possible efficiency between the same two reservoirs known now as the Carnot cycleAn engine operating in this cycle is called a Carnot engine. A the cycle is the second set of reactions in photosynthesis B the cycle release oxygen as a byproduct. A One complete Carnot cycle generates a vanishing change of entropy.
Such a proposition is. Check All That Apply Being a real cycle all processes in a Carnot cycle are irreversible. Textbook chapter 10 Irreversibilities are present in the Carnot Cycle.
Which is the incorrect statement about Carnot cycle. The boiler is not isothermal. According to Carnot Theorem.
DIt is a cycle modeled by four isentropic processes. An important feature of the Carnot cycle is that A its efficiency can be 100. It provides an upper limit on the efficiency that any classical thermodynamic engine can achieve during the conversion of heat into work or conversely the efficiency of a refrigeration system in creating a temperature.
C its efficiency is determined by the temperatures of the hot and cold reservoirs between which it works and by the properties of the working substance used and on nothing else. On the Carnot graph the area inside the cyle is equal to the work out for a. Also the efficiency of this type of engine is independent of the nature of the working substance and is only dependent on the temperature of the hot and cold reservoirs.
Which of the following statements is not true about the Calvin cycle. For a Carnot Cycle which of the following statements are true. A true b false Answer.
The condenser is not isothermal. A reversible cycle is an ideal hypothetical cycle in which all processes are reversible. The Carnot Cycle is the most efficient cycle operating between two specified temperature limits.
Solved A Which Of The Following Statements Are True For Chegg Com
The Carnot Cycle University Physics Volume 2
The Carnot Cycle University Physics Volume 2

Carnot S Perfect Heat Engine The Second Law Of Thermodynamics Restated Physics
The Carnot Cycle University Physics Volume 2
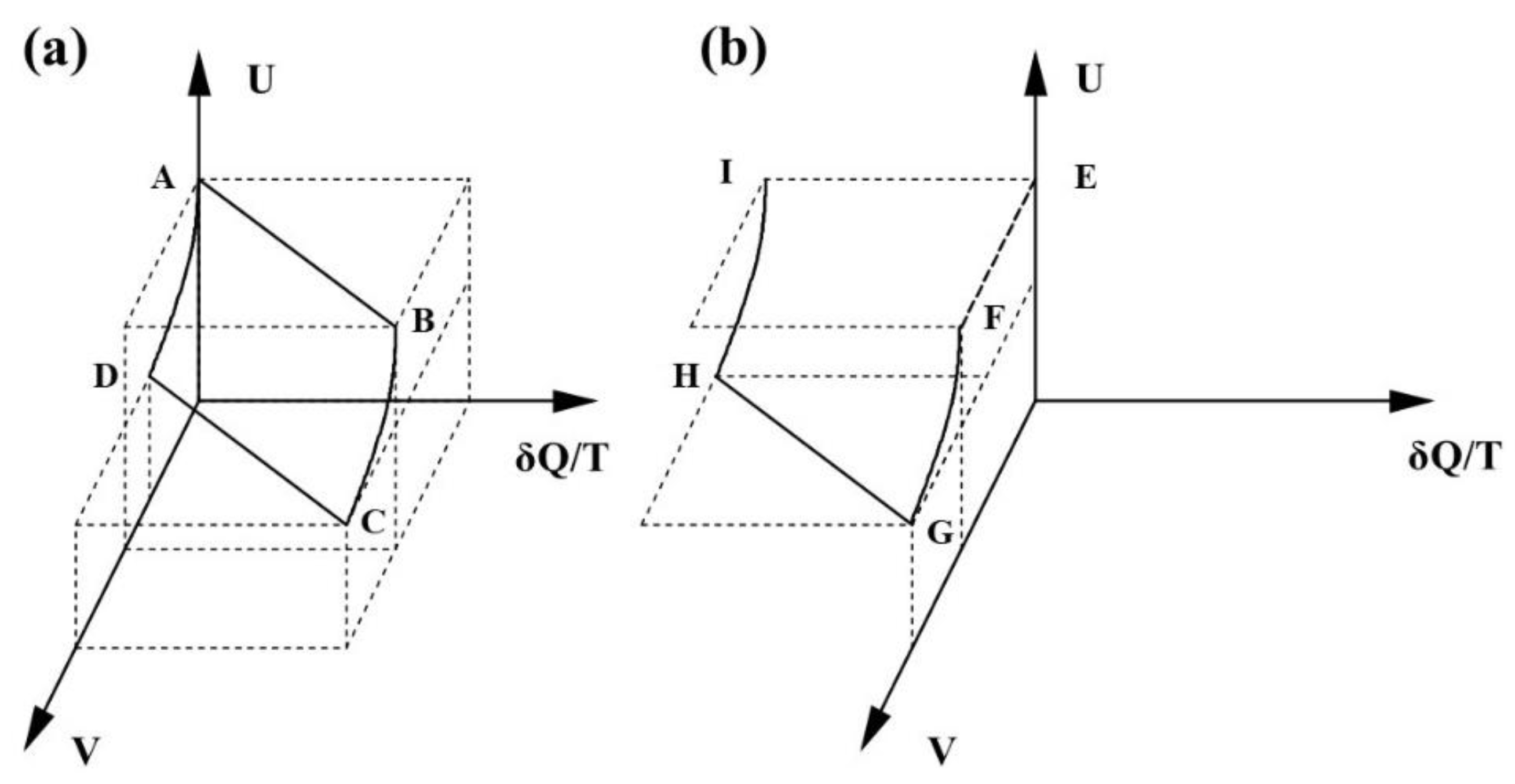
Entropy Free Full Text The Carnot Cycle Reversibility And Entropy Html

Carnot S Perfect Heat Engine The Second Law Of Thermodynamics Restated Physics

Carnot S Perfect Heat Engine The Second Law Of Thermodynamics Restated Physics

Carnot Cycle An Overview Sciencedirect Topics
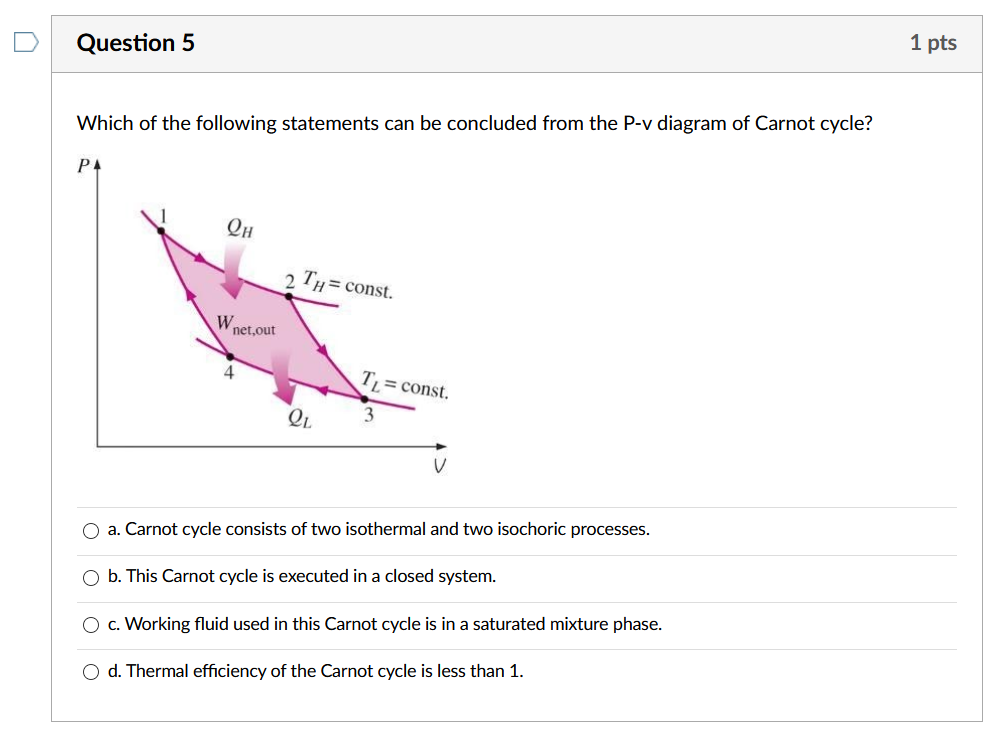
Solved Question 5 1 Pts Which Of The Following Statements Chegg Com

Solved Which Of The Following Statements About A Carnot Chegg Com
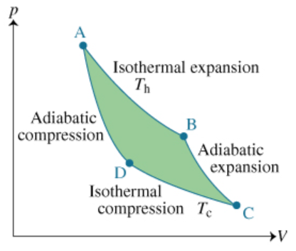
Solved A Which Of The Following Statements Are True Chegg Com

Entropy Free Full Text The Carnot Cycle Reversibility And Entropy Html

Carnot Cycle An Overview Sciencedirect Topics
Why Is The Carnot Engine The Most Efficient Quora

Carnot Efficiency 2 Reversing The Cycle Video Khan Academy

Carnot Cycle An Overview Sciencedirect Topics


Comments
Post a Comment